Typically, organic methods are shielded from the poisonous impact of free radicals by antioxidant protection. Extracts from orchids have been reported to point out excessive ranges of exogenous antioxidant exercise together with Bulbophyllum orchids however thus far, there have been no reviews on antioxidant enzymes.
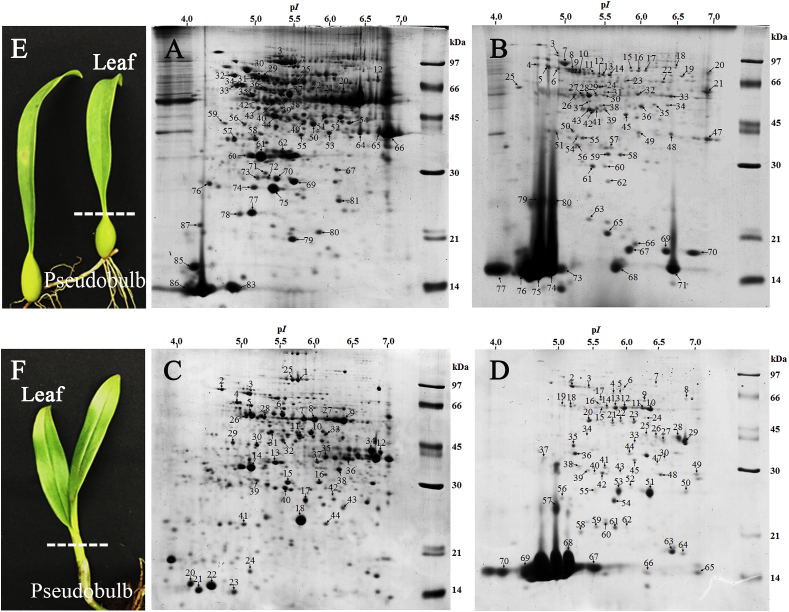
Therefore, variations in protein expression from leaves and pseudobulbs of Bulbophyllum morphologlorum Kraenzl and Dendrobium Sonia Earsakul had been studied utilizing two-dimensional gel electrophoresis and mass spectrometry (LC/MS/MS).
Interestingly, the most important group of these stress response proteins had been related to antioxidant protection and temperature stress, together with superoxide dismutase (Cu-Zn) and warmth shock protein 70. The excessive expression of this antioxidant enzyme from Bulbophyllum morphologlorum Kraenzl was confirmed by exercise staining on native-PAGE, and the 2 Cu/Zn-SODs isoenzymes had been recognized as Cu/Zn-SOD 1 and Cu/Zn-SOD 2 by LC/MS/MS.
The outcomes recommended that Bulbophyllum orchid is usually a potential plant supply for medicines and pure antioxidant dietary supplements.
Proteomic characterization of broken single hairs recovered after an explosion for protein-based human identification.
Evidence restoration is difficult the place an explosion has occurred. Though hair proof could also be sufficiently sturdy to be recovered on the web site, forensic evaluation underutilizes the matrix by counting on morphological evaluation. Where DNA is compromised, notably in hair, protein-based human identification presents a promising different.
Detection of amino acid polymorphisms in hair proteins as genetically variant peptides (GVPs) permits inference of individualizing single nucleotide polymorphisms for identification. However, an explosive blast might injury hair proteins and compromise GVP identification.
This work assesses results of an explosive blast on the hair proteome and GVP identification, investigates microscopy as a predictor of proteome profiling success in recovered hairs to enhance evaluation throughput, and quantifies discriminative energy in broken hairs. The proteomics dataset has been deposited into the ProteomeXchange Consortium (PXD017427).
With the exception of degradation in keratins Ok75, Ok80, Ok40, and keratin-associated protein KAP10-11 as markers of hair cuticular injury, corroborated by scanning electron microscopic evaluation, minimal hair proteome degradation following explosion allowed profitable proteome profiling of single hairs regardless of morphological injury.
Finally, GVP identification remained impartial of explosion circumstances, allowing comparable discriminative energy between exploded and undamaged hairs. These findings lend better confidence to GVP evaluation in one-inch hairs for forensic identification and present details about hair protein localization.